
Dear Colleagues,
Since we began our COVID-19 journey several months ago, not a day goes by without my appreciation for the tremendous dedication and persistence of our research community to understand this virus and its far-reaching implications on our communities, our cancer patients, and the world as we know it. I feel fortunate to work in an organization that is helping to advance COVID-related science in myriad areas, from the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) public-private partnership to independent testing and validation of antibody tests at the NCI Frederick National Laboratory for Cancer Research (FNLCR). Yet, I’m acutely aware of the unique family and work challenges that our community faces as we navigate this difficult and unpredictable course. I hope you are receiving the necessary support from our staff to continue your research.
We begin this issue with COVID-related information, funding opportunities, and resources. Instead of duplicating information you likely are already receiving from other sources, we encourage you to bookmark two NIH and NCI websites dedicated to researchers. We do, however, highlight specific resources such as the COVID-19 Portfolio Tool and the Survey Items available through PhenX.
In this issue you’ll also find new funding opportunities and notices of special interest (NOSIs) related to multiple morbidities and the link between alcohol and cancer, among others. Additionally, tobacco control researchers will be excited to have access to newly released 2018–2019 data from the Tobacco Use Supplement to the Current Population Survey (TUS-CPS) that include new topics such as workplace and home vaping restrictions, e-cigarette price, and prevalence of heated tobacco product use. Other recently updated resources include the Cancer Trends Progress Report, the Annual Report to the Nation on the Status of Cancer, and the SEER Cancer Statistics Review (CSR), 1975–2017.
Finally, we are pleased to welcome new staff to our program, as well as say farewell to others who are transitioning to other areas in NCI.
As a reminder, you’re free to manage your BRP newsletter subscription at any time, but we hope you find the information beneficial. If you’re on social media, follow us on Twitter at @NCIBehaviors. ![]()
Funding Opportunities
New Funding Opportunity Announcements
Tobacco Use and HIV in Low and Middle-Income Countries
U01 Clinical Trial Optional; RFA-CA-20-037
NCI Contact: Dr. Mark Parascandola
Posted: July 7, 2020
Expires: September 25, 2020
Interventions to Prevent Electronic Nicotine Delivery Systems (ENDS) Use Among Adolescents
R01 Clinical Trial Optional; RFA-DA-21-009
BRP Contact: Dr. Rachel Grana Mayne
Posted: June 16, 2020
Expires: October 20, 2020
Advancing Research to Develop Improved Measures and Methods for Understanding Multimorbidity
R01 Clinical Trial Optional; PAR-20-179
NCI Contact: Dr. Bryan Kim
Posted: April 30, 2020
Expires: September 8, 2023
Identifying Innovative Mechanisms or Interventions that Target Multimorbidity and Its Consequences
R01 Clinical Trial Optional; PAR-20-180
NCI Contact: Dr. Bryan Kim
Posted: April 30, 2020
Expires: September 8, 2023
Mid-Career Enhancement Awards to Integrate Basic Behavioral, Biomedical, and/or Social Scientific Processes
K18 Basic Experimental Studies with Humans Required; PAR-20-226
K18 No Independent Clinical Trials; PAR-20-211
NCI Contact: Ms. Gina Tesauro
Posted: June 3, 2020
Expires: March 18, 2023
Reissuances
Improving Smoking Cessation Interventions among People Living with HIV
R01 Clinical Trial Optional; RFA-CA-20-035
R21 Clinical Trial Optional; RFA-CA-20-036
BRP Contact: Dr. Annette Kaufman
Posted: June 10, 2020
Expires: September 5, 2020
Notices of Special Interest (NOSIs)
NCI Emergency Administrative Supplements for Research and Training Continuity of Postdoctoral Fellows during Coronavirus Disease 2019 (COVID-19)
NOT-CA-20-082
Posted: July 6, 2020
Expires: August 15, 2020
Research to Advance the Understanding of Tobacco Product Pharmacokinetic Research
NOT-OD-20-081
BRP Contact: Dr. Rachel Grana Mayne
Posted: March 25, 2020
Expires: February 13, 2021
Development and Preliminary Testing of Health-related Behavioral Interventions
NOT-OD-20-106
BRP Contact: Dr. Susan Czajkowski
Posted: May 19, 2020
Expires: September 26, 2022
Research on Oral Anticancer Agents in the Contexts of Utilization, Adherence, and Health Care Delivery
NOT-CA-20-026
BRP Contact: Dr. Wendy Nelson
Posted: February 21, 2020
Expires: May 8, 2023
Advancing Cancer Data Repositories and Knowledgebases
NOT-CA-20-045
NCI Contact: Dr. Juli Klemm
Posted: April 9, 2020
Expires: May 9, 2023
Alcohol and Cancer Control
NOT-CA-20-034
BRP Contacts: Dr. Tanya Agurs-Collins and Dr. David Berrigan
Posted: March 18, 2020
Expires: September 9, 2023
COVID-19 Resources and Information
The NIH and NCI web pages, COVID-19 resources for NIH applicants and recipients and COVID-19 resources for cancer researchers, are being updated frequently. Bookmark these pages and come back to them often to stay informed about COVID-19 specific funding opportunities, resources, FAQs, and more. You may also consider subscribing to the NIH Extramural Nexus monthly update and the Open Mike blog for additional information from NIH. Subscribe to the newsletter »
NIH has a dedicated web page listing all funding opportunities specific to COVID-19. View COVID-related funding »
The NIH Office of Behavioral and Social Sciences Research provides a curated list of COVID-19 funding opportunities for behavioral and social sciences. View COVID-19 behavioral and social science funding opportunities »
Smokefree.gov announces new COVID-19-related smoking cessation content

The Smokefree.gov Initiative has released a new web page, Smoking and COVID-19, to assist current and former smokers who are concerned about their health and are seeking evidence-based smoking cessation information and tools. As the novel coronavirus has spread globally, current or former smokers may be at increased risk of infection with the virus that causes COVID-19, and they may have worse outcomes from COVID-19. Key content provides guidance for quitting in the context of this increased concern. It also acknowledges the new or increased anxiety or depression people may be experiencing as a result of the socioeconomic impacts of the pandemic that may impede quitting or trigger a return to smoking. View the Smoking and COVID-19 webpage »
COVID-19 Portfolio tool now available
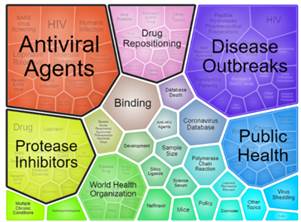 Figure: Interactive foam tree visualization
Figure: Interactive foam tree visualization
NIH has assembled a comprehensive portfolio of COVID‑19 publications and preprints that is freely available to the public and coupled it with a user-friendly portfolio analysis interface for querying the full text and supplemental data. The COVID-19 portfolio is updated daily with new literature selected for inclusion by subject matter experts. The tool also includes an interactive visualization feature that groups articles into clusters based on key terms, allowing users an at-a-glance view of their search topics. Explore the COVID-19 Portfolio »
COVID-19 specific survey items now available on PhenX and the NIH Disaster Research Response (DR2) platforms
For researchers interested in assessing COVID-19 specific domains, NIH is making existing COVID-19 survey items and investigator contact information available via two NIH-supported survey item platforms: 1) NIH Public Health Emergency and Disaster Research Response (DR2) and 2) PhenX Toolkit ![]() (consensus measures for Phenotypes and eXposures). The goal is to minimize the proliferation of one-off survey items, encourage comparisons across samples, and facilitate data integration and collaboration. Researchers addressing COVID-19 questions, whether population-based or for clinical research, are encouraged to consider these COVID-19 specific survey item repositories and select existing survey items or protocol modules currently being fielded. Researchers with additional survey items about to be fielded are encouraged to make them public for other researchers to consider by submitting the survey to NIHCOVID19Measures@nih.gov.
(consensus measures for Phenotypes and eXposures). The goal is to minimize the proliferation of one-off survey items, encourage comparisons across samples, and facilitate data integration and collaboration. Researchers addressing COVID-19 questions, whether population-based or for clinical research, are encouraged to consider these COVID-19 specific survey item repositories and select existing survey items or protocol modules currently being fielded. Researchers with additional survey items about to be fielded are encouraged to make them public for other researchers to consider by submitting the survey to NIHCOVID19Measures@nih.gov.
View the DR2 Platform and PhenX Toolkit ![]() .
.
Implementation of a statewide hotline system in response to COVID-19
A recent paper led by BRP grantee Dr. Jennifer DeVoe describes the challenges primary care practices are facing due to the COVID-19 pandemic and the urgent need for collaboration between academic health centers and state and local public health agencies to alleviate the burden on the primary health care system. Oregon Health & Science University has partnered in this way to implement the centralized COVID-19 Connected Care Center. The three-phase implementation approach includes a statewide hotline that was made available to established Oregon Health & Science University patients, allowing for immediate nursing assessment and facilitation of primary care telemedicine visits (Phase I). Phases II and III involve expanding the hotline format to primary care practices throughout the state and, eventually, to the 25% of Oregonians without primary care access and to patients of small practices that lack the resources of larger health care systems. BRP grantee Dr. Alex Krist is also a co-author on the paper.
DeVoe J.E., Cheng A., Krist A. Regional strategies for academic health centers to support primary care during the COVID-19 pandemic: A plea from the front lines ![]() . JAMA Health Forum (2020).
. JAMA Health Forum (2020).
NIH Preprint Pilot launched to disseminate COVID-19 research
The National Library of Medicine recently launched the NIH Preprint Pilot to help accelerate the dissemination of research on SARS-CoV-2 virus and COVID-19. Preprints are complete, public drafts of scientific documents that have not yet undergone peer review. The pilot will run for at least 12 months and test the feasibility of making preprints that report NIH-supported COVID-19 research searchable in PubMed Central and PubMed.
New NIH analytics platform of nationwide COVID-19 patient data
NIH has launched a centralized and secure analytics platform to systematically collect large amounts of clinical, laboratory, and diagnostic data on people diagnosed with COVID-19 via health care provider organizations nationwide. The data will be harmonized into a standard format and made available to researchers and clinicians to accelerate COVID-19 research and improve clinical care. This is part of the National COVID Cohort Collaborative (N3C) effort, which aims to turn clinical information into knowledge needed to study COVID-19, including health risk factors related to disease outcomes, and identify potentially effective treatments. Learn more »
NCI launches a natural history study to understand COVID-19’s impact on cancer patients
In May, NCI launched the NCI COVID-19 in Cancer Patients Study, or NCCAPS, a natural history study designed to help scientists answer key questions about the impact of COVID-19 on cancer patients. Specifically, researchers aim to learn more about the risk factors related to serious illness from COVID-19, the effect of COVID-19 on cancer treatment, and genetic risk factors and markers of serious illness from COVID-19. Additionally, they will create a bank of data, blood samples, and images from people with COVID-19 and cancer for future research. NCI hopes to enroll 2,000 patients and is encouraging participation from NCI-funded clinical trial sites. Learn more about NCCAPS »
NIH-Wide Strategic Plan for COVID-19 Research now available
The NIH-wide COVID-19 Strategic Plan provides a framework for accelerating the development of therapeutic interventions, vaccines, and diagnostics. NIH will implement five cross-cutting strategies to increase fundamental knowledge about the virus and the disease, accelerate innovation in testing technologies, speed the use of promising therapeutics and vaccines, identify effective approaches to promote individual and community safety, and ensure accessibility of diagnostic, treatment and prevention options to underserved and vulnerable populations. Read the strategic plan »
Scientific Advances
ENDS research messages and health care professionals
BRP grantees Dr. Olivia Wackowski, Dr. Christine Delnevo, Dr. Michael Steinberg, and colleagues examined medical journal articles (2012–2018) to assess topics covered, terminology, and risk or benefit statements related to electronic nicotine delivery systems (ENDS). Negative statements changed over time from harmful chemical exposure (“toxins and carcinogens”) to appeal to youth or serve as a gateway to other tobacco products. Few articles (12.6%) included guidance for health care professionals. Future research may consider exploring the variety of information sources that influence clinicians’ attitudes toward ENDS.
Briganti M., Wackowski O.A., Delnevo C.D., Brown L., Hastings S.E., Singh B., Steinberg M.B. Content analysis of electronic nicotine delivery system publications in core clinical journals from 2012 to 2018. Int J Environ Res Public Health (2020).
Establishing the effectiveness of implementation strategies for smoking cessation services in lung cancer screening imaging facilities
A new publication reports the study design and evaluation plans for a national cluster randomized trial, led by BRP grantees Drs. Kristie Foley and Caroline Chiles, testing strategies for the implementation of smoking cessation services for long-term smokers in low-dose computed tomography (LDCT) lung cancer screening imaging facilities. In the Optimizing Lung Screening (OaSiS) trial, 26 National Community Oncology Research Program (NCORP) sites across the United States were randomly assigned to either a compendium of implementation strategies to add or enhance smoking cessation services during lung cancer screening, or to usual care. The OaSiS trial is part of NCI’s Smoking Cessation at Lung Examination (SCALE) Collaboration, which was established for investigators to engage in cross-project research by sharing data and methods from eight ongoing trials of smoking cessation interventions for long-term smokers at LDCT screening sites.
Foley K.L., Miller D.P. Jr., Weaver K., Sutfin E.L., Petty W.J., Bellinger C., Spangler J., Stone R.J., Lawler D., Davis W., Dressler E., Lesser G., Chiles C. The OaSiS trial: A hybrid type II, national cluster randomized trial to implement smoking cessation during CT screening for lung cancer. Contemp Clin Trials (2020).
Genome-wide association study of biomarkers for nicotine metabolism may advance the epidemiology of tobacco-related disease and inform smoking cessation strategies
A research team, including BRP grantee Dr. Caryn Lerman, performed a large genome-wide association study of the nicotine metabolite ratio (NMR), a biomarker for nicotine clearance, in current smokers of European descent. The researchers identified 1,255 genome-wide significant variants, as well as 14 putatively causal single-nucleotide polymorphisms (SNPs), which together explain nearly 40% of NMR variation. Understanding the genetic factors influencing smoking-related traits facilitates epidemiological studies of smoking and disease and may inform precision medicine approaches to smoking cessation treatment.
Buchwald J., Chenoweth M.J., Palviainen T., Zhu G., Benner C., Gordon S., Korhonen T., Ripatti S., Madden P.A.F., Lehtimäki T., Raitakari O.T., Salomaa V., Rose R.J., George T.P., Lerman C., Pirinen M., Martin N.G., Kaprio J., Loukola A., Tyndale R.F. Genome-wide association meta-analysis of nicotine metabolism and cigarette consumption measures in smokers of European descent. Mol Psychiatry (2020).
Strong snack laws associated with higher vegetable consumption among high school students
A recent paper led by BRP grantee Dr. Jamie Chriqui examined the association between state snack laws and high school student fruit and vegetable consumption pre- and post-federal Smart Snacks standards (effective school year 2014–2015). Researchers used 2004–2016 data from NCI’s Classification of Laws Associated with School Students (CLASS) and 2005–2017 Youth Risk Behavior Survey data. This study provides evidence that students in states with strong snack laws had higher odds of consuming vegetables—salad, in particular—compared to students in states with no laws. Findings show the important role that standards restricting the availability of junk foods in schools play on increasing student vegetable consumption. Additionally, the findings illustrate how federal standards harmonized the patchwork of state laws that existed prior to Smart Snacks and the important role of consistent national standards in supporting student vegetable consumption. BRP Program Director Dr. Frank Perna was a co-author on the paper.
Chriqui J.F., Lin W., Leider J., Shang C., Perna F.M. The harmonizing effect of Smart Snacks on the association between state snack laws and high school students' fruit and vegetable consumption, United States-2005-2017. Prev Med (2020).
Exercise therapy dosing schedule on impaired cardiorespiratory fitness in breast cancer patients
A research team including BRP grantee Dr. Lee Jones conducted a randomized controlled trial to examine the tolerability and efficacy of two exercise training dose regimens on cardiorespiratory fitness and patient-reported outcomes in patients with posttreatment primary breast cancer. The main outcome was change in peak oxygen consumption from baseline to after the intervention. Secondary outcomes included patient-reported outcomes, tolerability, and safety. Their findings indicate that short-term exercise training, independently of dosing schedule, is associated with modest improvements in cardiorespiratory fitness in patients previously treated for early-stage breast cancer.
Scott J.M., Thomas S.M., Peppercorn J.M., Herndon J.E. 2nd, Douglas P.S., Khouri M.G., Dang C.T., Yu A.F., Catalina D., Ciolino C., Capaci C., Michalski M.G., Eves N.D., Jones L.W. Effects of exercise therapy dosing schedule on impaired cardiorespiratory fitness in patients with primary breast cancer: A randomized controlled trial. Circulation (2020).
Neighborhood environment attributes strongly related to physical activity outcomes and obesity
A recent paper led by Dr. Jim Sallis features the IPEN (International Physical Activity and Environment Network) Adult Study, which was conducted to provide international evidence on associations of built environments with physical activity and weight status in twelve countries on five continents. The research team reanalyzed data from eight primary papers to identify patterns of findings across studies. Their findings indicate that neighborhood environment attributes (objectively measured and self-reported) were strongly related to all physical activity outcomes (accelerometer-assessed total physical activity, reported walking for transport and leisure) and meaningfully related to overweight/obesity. Overall, findings provide evidence in support of global initiatives to increase physical activity and control noncommunicable diseases while achieving sustainable development goals. Co-authors include current and former BRP grantees.
Sallis J.F., Cerin E., Kerr J., Adams M.A., Sugiyama T., Christiansen L.B., Schipperijn J., Davey R., Salvo D., Frank L.D., De Bourdeaudhuij I., Owen N. Built environment, physical activity, and obesity: Findings from the International Physical Activity and Environment Network (IPEN) adult study. Annu Rev Public Health (2020).
Research and resource needs identified by the second Cancer and Accelerated Aging think tank
Last year, the NCI Cancer and Accelerated Aging initiative hosted a think tank to advance progress in the area of accelerated aging among adult survivors of childhood cancer. Experts convened and shared promising strategies to prevent, slow, or reverse the aging consequences of cancer and its treatment. The meeting identified multiple research and resource needs including geroscience-guided clinical trials and comprehensive assessments of functional, cognitive, and psychosocial challenges, among others. The paper below summarizes the meeting outcomes and was led by BRP fellow Dr. Jennifer Guida; co-authors included several NCI staff and grantees.
Guida J.L., Agurs-Collins T., Ahles T.A., Campisi J., Dale W., Demark-Wahnefried W., Dietrich J., Fuldner R., Gallicchio L., Green P.A., Hurria A., Janelsins M.C., Jhappan C., Kirkland J.L., Kohanski R., Longo V., Meydani S., Mohile S., Niedernhofer L.J., Nelson C., Perna F., Schadler K., Scott J.M., Schrack J.A., Tracy R.P., van Deursen J., Ness K.K. Strategies to prevent or remediate cancer and treatment-related aging. J Natl Cancer Inst (2020).
Results from a phase 2 randomized controlled biomarker trial among individuals receiving hematopoietic cell transplant for multiple myeloma
In a recent paper, researchers conducted a randomized controlled phase 2 biomarker trial testing the impact of the nonselective β-antagonist propranolol on conserved transcriptional response to adversity (CTRA)-related gene expression of 25 individuals receiving an autologous hematopoietic cell transplant (HCT) for multiple myeloma. Findings indicate that peri-HCT propranolol inhibits cellular and molecular pathways associated with adverse outcomes. Changes in these pathways make propranolol a potential candidate for adjunctive therapy in cancer-related HCT. This is the initial report from an NCI Network on Biobehavioral Pathways in Cancer project awarded to Wisconsin College of Medicine, Dr. Jennifer Knight (PI), through the NCI prime contract with Leidos.
Knight J.M., Rizzo J.D., Hari P., Pasquini M.C., Giles K.E., D’Souza A., Logan B.R., Hamadani M., Chhabra S., Dhakal B., Shah N., Sriram D., Horowitz M.M., Cole S.W. Propranolol inhibits molecular risk markers in HCT recipients: A phase 2 randomized controlled biomarker trial. Blood Adv (2020).
Examining cancer-related cognitive impairment and a genetic risk factor for Alzheimer’s disease using a mouse model
The genetic factor APOE4 is the strongest genetic risk factor for Alzheimer's disease. A paper including BRP grantee Dr. Jeanne Mandelblatt examined whether the relationship between APOE4 and cancer-related cognitive impairment can be demonstrated in a mouse model to identify associations of chemotherapy with behavioral and structural correlates of cognition, and to test whether chemotherapy affects markers of Alzheimer's disease. Findings indicate cognitive impairments in aged APOE4 knock-in mice after doxorubicin treatment and establish this system as a novel and powerful model of cancer-related cognitive impairment.
Demby T.C., Rodriguez O., McCarthy C.W., Lee Y., Albanese C., Mandelblatt J., Rebeck G.W. A mouse model of chemotherapy-related cognitive impairments integrating the risk factors of aging and APOE4 genotype. Behav Brain Res (2020).
Perception of one’s cancer risk and why it matters for clinical oncology
BRP Associate Director Dr. William Klein and BRP Program Directors Dr. Rebecca Ferrer and Dr. Annette Kaufman recently published a commentary that discusses how people reflect on their cancer risk and the implications for clinical oncology. The assumption that people actually “think” about risk is not fully supported by decision science research. Instead, individuals’ risk perceptions may be influenced by social factors, along with emotional and motivational factors. Implications for clinical oncology include acknowledging that patients and the general population’s understanding and reaction to risk are rooted in emotion, intuition, social comparisons and potentially reflecting an adaptive orientation toward risk. In turn, recipients of risk messages may be particularly responsive to practices and interventions that highlight social comparisons, quell defensiveness, and acknowledge negative emotions.
Klein W.M.P., Ferrer R.A., Kaufman A.R. How (or do) people “think” about cancer risk, and why that matters. JAMA Oncol (2020).
Assessing simulated cancer prevention Facebook messages using eye-tracking
In a recent paper, researchers conducted a mixed-methods experimental study that used eye-tracking technology to examine social media users’ attention to simulated cancer prevention Facebook posts. Stimuli conditions included message format (narrative/non-narrative), information veracity, source (organization/individual), and cancer topic (HPV prevention and sunscreen safety). Overall, users spent significantly more time on posts attributed to individuals and on narrative-based posts. Additionally, users spent approximately equal amounts of time focusing on the source of the message as the content. There were no significant differences in the amount of time spent on the other message components by condition. The paper was led by BRP Program Director Dr. Sylvia Chou, and co-authors included current and former BRP fellows and collaborators.
Chou W.S., Trivedi N., Peterson E., Gaysynsky A., Krakow M., Vraga E. How do social media users process cancer prevention messages on Facebook? An eye-tracking study. Patient Educ Couns (2020).
Latinas’ reported knowledge and perceptions of genetic cancer risk assessment
A research team including BRP grantees Dr. Vanessa Sheppard and Dr. Alejandra Hurtado-de-Mendoza conducted a qualitative study and interviewed 20 Latinas (10 affected, 10 unaffected) at increased risk for hereditary breast and ovarian cancer. They asked participants about their knowledge and perceptions of genetic cancer risk assessment, hereditary breast and ovarian cancer, risk, benefits, motivators, barriers, challenges, and experiences with genetic cancer risk assessment. Findings identified multiple barriers and facilitators to genetic cancer risk assessment uptake in this population, including patient-level psychosocial/cultural factors (e.g., limited knowledge, worry about relatives' risk) and health care system factors (e.g., receiving no referrals). Additionally, there were notable differences in awareness and knowledge between affected and unaffected women (e.g., genetic testing awareness), as well as knowledge gaps that were evident in both groups (e.g., age of diagnosis as a risk factor).
Gómez-Trillos S., Sheppard V.B., Graves K.D., Song M., Anderson L., Ostrove N., Lopez K., Campos C., Gonzalez N., Hurtado-de-Mendoza A. Latinas' knowledge of and experiences with genetic cancer risk assessment: Barriers and facilitators. J Genet Couns (2019).
Determining patterns of mobile health technology use and behavioral tracking among US adults
BRP fellow Dr. Camella Rising led a paper that used NCI Health Information National Trends Survey (HINTS) data to examine patterns of mobile health (mHealth) technology use for health and behavioral tracking among 6,789 US adults. They characterized respondents into five patterns of mHealth use on a spectrum from mHealth nonowners and nonusers to supertrackers who track health or behaviors and goals using a smartphone or tablet plus other devices. Researchers found distinctive sociodemographic and health-related characteristics of the population by pattern of mHealth use, with noticeable differences between individuals who do and do not use devices to track goals. Several characteristics of individuals who track health or behaviors but not goals (device trackers) are similar to those of mHealth nonowners and nonusers. Results indicate that compared with supertrackers, device trackers have higher odds of being male, are older in age, have an annual household income of US $20,000 to US $49,999, and have a chronic health condition. Device trackers also have higher odds of not being health information seekers than supertrackers. Co-authors include Dr. Roxanne Jensen, Program Director in the DCCPS Healthcare Delivery Research Program; Dr. Richard Moser, BRP Research Methods Coordinator; and Dr. April Oh, Senior Advisor for DCCPS' Implementation Science and Health Equity.
Rising C.J., Jensen R.E., Moser R.P., Oh A. Characterizing the US population by patterns of mobile health use for health and behavioral tracking: Analysis of the National Cancer Institute's Health Information National Trends Survey data. J Med Internet Res (2020).
In the News
How researchers are helping cancer survivors cope with cancer-related anxiety and distress
A recent NCI Cancer Currents blog post highlights different approaches being employed to help cancer survivors manage cancer-related anxiety and distress and features the work of BRP grantees Dr. Wonsun (Sunny) Kim and Dr. Kerri Winters-Stone. Dr. Kim is studying the effectiveness of a 4-week web-based digital storytelling approach to help both patients with cancer undergoing hematopoietic stem cell transplantation and their caregivers. Dr. Winters-Stone is studying the effects of partnered exercise training on the physical and mental health and relationship quality of couples (survivors and their partners) coping with cancer.
Staffing News
Dr. April Oh has transitioned from her role as a Program Director in the Health Communication and Informatics Research Branch to become a Senior Advisor for Implementation Science and Health Equity as part of the DCCPS Implementation Science team. In this role, Dr. Oh will advance research in implementation science and health equity while continuing to provide leadership for the Implementation Science Centers in Cancer Control (ISC3).
Dr. Maria Roditis joined the Tobacco Control Research Branch as a Program Director in June. Dr. Roditis joined NCI from FDA’s Center for Tobacco Products/Office of Health Communication and Evaluation, where she led formative research efforts for FDA’s “The Real Cost” Cigarette and ENDS campaigns. While at FDA, Dr. Roditis also served as co-lead of formative research for “The Real Cost” Smokeless campaign and for FDA’s first adult smoking cessation campaign, “Every Try Counts.” As a postdoctoral fellow, Dr. Roditis trained with both Dr. Bonnie Halpern-Felsher and Dr. Stanton A. Glantz, where her research focused on adolescent perceptions of addiction, risks, and benefits related to tobacco use. Dr. Roditis received her PhD from Indiana University (IU) Bloomington with a focus in Anthropology, her MPH also from IU, and her BA from the University of Illinois Urbana-Champaign in Anthropology.
Dr. Mark Parascandola has transitioned to the NCI Center for Global Health (CGH) as the Branch Chief for Research and Training. He served in this role in an acting capacity for the past year and a half while also serving as a Program Director in the Tobacco Control Research Branch. Dr. Parascandola will continue to manage a portfolio of international tobacco control research grants.
Resources
2018–2019 TUS-CPS data now publicly available

Data from the 2018–2019 Tobacco Use Supplement to the Current Population Survey (TUS-CPS)—including July 2018, January 2019, and May 2019 data—are now publicly available. New topics for 2018–2019 include workplace and home vaping restrictions, e-cigarette price, and prevalence of heated tobacco product use. An accompanying Data Brief highlighting key findings, as well as the data files, SAS program code, and other technical documentation, is available. The TUS-CPS web pages have also been recently updated to include the latest publications using TUS-CPS data, as well as a 2020 Informational Session Questions and Responses document featuring detailed responses by NCI TUS-CPS staff to technical questions submitted by researchers nationwide. Access the data » View the data brief »
Updated Cancer Trends Progress Report now available
The NCI Cancer Trends Progress Report has recently been updated. This report offers national trend data across many cancer control measures. The data are displayed in relation to Healthy People 2020 targets, where they are available. View measures and generate custom reports »
Declines in overall cancer mortality highlighted in recent Annual Report to the Nation on the Status of Cancer
The recently released Annual Report to the Nation on the Status of Cancer showed that overall cancer death rates decreased 1.5% on average per year from 2001 to 2017, decreasing more rapidly among men than among women. The report also indicated that Healthy People 2020 targets were met for lung, colorectal, and prostate cancer death rates. The annual report is a collaborative effort among the Centers for Disease Control and Prevention, NCI, the American Cancer Society, and the North American Association of Central Cancer Registries. View the details of the report »
PhenX toolkit for tobacco regulatory research
Common measures and data elements for use in tobacco regulatory research (TRR) are now available as part of the PhenX toolkit. The goal of the TRR toolkit is to improve data comparability and to facilitate cross-study analyses and replication of findings in human subjects to inform the regulatory activities of the US Food and Drug Administration Center for Tobacco Products. Access the TRR Collection » ![]() . Read more about the work
. Read more about the work ![]() of the scientific panel that oversaw the TRR Core Collection of measures development.
of the scientific panel that oversaw the TRR Core Collection of measures development.
All of Us Researcher Workbench for beta testing
NIH is accepting applications from researchers to use and provide feedback on its beta version of the All of Us Researcher Workbench platform, suite of custom tools, and protected data set. Researchers can access Registered Tier data, workspaces, the Cohort Builder tool, interactive Jupyter Notebooks, and more. Over time, All of Us will make available COVID-19 related data from antibody testing, a survey on the pandemic’s impacts, and collection of electronic health record information. To register, the researcher’s organization must have an institutional agreement with the All of Us Research Program. Researchers must also have an eRA Commons account and complete the All of Us Research Program data access process to access the Researcher Workbench and Registered Tier data. Apply to be an All of Us Researcher » ![]()
SRNT-University (SRNT-U) podcast—Tobacco Use After a Cancer Diagnosis
NCI Program Director Dr. Stephanie Land partnered with the Society for Research on Nicotine & Tobacco (SRNT) to create the podcast series “Tobacco Use After a Cancer Diagnosis” ![]() for SRNT University. Each podcast features an interview with a tobacco control researcher, with topics ranging from the biology of nicotine exposure and cancer to the health care cost attributable to continued tobacco use after cancer diagnosis. To date, interviews feature the following experts:
for SRNT University. Each podcast features an interview with a tobacco control researcher, with topics ranging from the biology of nicotine exposure and cancer to the health care cost attributable to continued tobacco use after cancer diagnosis. To date, interviews feature the following experts:
Dr. Graham Warren (Medical University of South Carolina)
Dr. Monica Webb-Hooper (National Institute on Minority Health and Health Disparities)
Dr. Donna Shelley (New York University)
Dr. Robert Schnoll (University of Pennsylvania)
Dr. Scott Weed (West Virginia University)
Dr. Pankaj Chaturvedi (Tata Memorial Hospital Mumbai, India)
Dr. Michael Fiore (University of Wisconsin)
New episodes are added regularly and will feature Dr. Mohan Devbhandari (Bhaktapur, Nepal) and Dr. Srikumar Chellappan (Moffitt Cancer Center).
CPSTF recommends internet-based interventions for tobacco use cessation
The Community Preventive Services Task Force (CPSTF) recommends internet-based interventions to increase tobacco use cessation. Evidence shows these interventions increase cessation among adults interested in quitting when measured six or more months following intervention. Following an evaluation of McCrabb et al.’s 2019 systematic review and meta-analysis, the CPSTF issued this recommendation update to replace the 2011 CPSTF finding of insufficient evidence for this intervention approach. Read more about the updated recommendation » ![]()
SEER releases new Cancer Statistics Review and latest SEER data
NCI’s Surveillance Research Program recently released the SEER Cancer Statistics Review (CSR), 1975–2017. The updated Cancer Statistics Review presents the most recent cancer incidence, mortality, survival, and prevalence statistics. Researchers can also obtain new data and statistics from the November 2019 SEER submission and the latest US mortality file. Get the Cancer Statistics Review and the SEER Data »
ADOPT environmental measures now available on new NCI GIS Portal for Cancer Research
The NHLBI- and NCI-led Accumulating Data to Optimally Predict obesity Treatment (ADOPT) project resulted in the development of a set of recommended measures to further understand variation in responses to weight loss interventions. A new data resource facilitating use of the environmental measures recommended by the ADOPT team is now available on the NCI GIS Portal for Cancer Research. The environmental domain web resource presents and describes methods to facilitate use of area-level environmental constructs concerning walkability, personal safety, urbanicity, and more. Methods are described to obtain safety and food environment related variables. These data layers should facilitate analysis of whether environmental features moderate responses to weight loss interventions. Explore data for ADOPT environmental constructs »
NCCOR’s Youth Compendium of Physical Activities now available in Spanish

The National Collaborative on Childhood Obesity Research (NCCOR’s) Youth Compendium of Physical Activities is now available in Spanish. The compendium provides a list of 196 common activities in which youth participate and the estimated energy cost associated with each activity. The Spanish version includes activities common in Spain, Mexico, and Colombia. It can be used by a wide variety of people—from researchers and health care professionals to teachers, coaches, and fitness professionals—and in a variety of ways—including research, education, and interventions to encourage physical activity in youth. Access the Spanish-language Youth Compendium of Physical Activities » ![]()
NIH releases strategic plan for NIH nutrition research
NIH, guided by its Nutrition Research Task Force (NRTF), released its 2020–2030 Strategic Plan for NIH Nutrition Research to direct its vision to advance nutrition science discoveries over the next 10 years. With a focus on precision nutrition, the plan emphasizes cross-cutting, innovative opportunities to cultivate nutrition research across a wide range of areas, from basic science to experimental design to research training. Learn more »
Scientific Report of the 2020 Dietary Guidelines Advisory Committee now available
The Scientific Report of the 2020 Dietary Guidelines Advisory Committee is now available to the public. Researchers and the general public are encouraged to provide feedback on the report to the United States Departments of Agriculture (USDA) and Health and Human Services (HHS) by August 13, 2020 at 11:59 p.m. ET. USDA and HHS will hold a virtual meeting on August 11, 8:30am-1:00 p.m. ET, to hear public comments on the Scientific Report. Download the Scientific Report »
Awards and Recognitions

Basic Biobehavioral and Psychological Sciences Branch Branch Chief Dr. Paige Green was recently elected as the 2021 President of the American Psychosomatic Society ![]() , an organization dedicated to advancing and integrating the scientific study of biological, psychological, behavioral, and social factors in health and disease.
, an organization dedicated to advancing and integrating the scientific study of biological, psychological, behavioral, and social factors in health and disease.

BRP fellow Dr. Sydney O’Connor was selected as the recipient of the 2020 Society of Behavioral Medicine Dissertation Award. Her dissertation was titled “Sleep health and variability in youth: a real-time data capture study to examine influences on daily dietary intake patterns and longitudinal weight trajectories.” She also was one of several awardees of the NIH Fellows Award for Research Excellence (FARE) 2021 competition. Dr. O’Connor’s abstract was titled “Circadian timing of eating and weight status among adults in the American Time Use Survey.”
BRP fellow Dr. Neha Trivedi also received an NIH Fellows Award for Research Excellence (FARE) for her abstract, “Understanding information processing and perceived message believability of simulated Facebook cancer-related posts: findings from a mixed methods eye-tracking study.”

BRP Program Director Dr. Gordon Willis received the 2020 Public Service Award by the American Association for Public Opinion Research ![]() . The award honors public sector employees who have contributed to the quality of government surveys, data systems, research, and/or leadership.
. The award honors public sector employees who have contributed to the quality of government surveys, data systems, research, and/or leadership.

BRP Program Director Dr. David Berrigan was appointed to the National Academies of Science, Engineering, and Medicine’s Transportation Research Board ![]() . The Board’s mission is to promote innovation and progress in transportation through research.
. The Board’s mission is to promote innovation and progress in transportation through research.
Six BRP fellows selected as CRAFT awardees
The Cancer Research Award for Fellows in Training (CRAFT) initiative provides funding for DCCPS fellows to conduct primary data collection using NCI’s Human Systems Integration lab. Of the twelve 2020 award recipients, six are fellows in the Behavioral Research Program.
| Principal Investigator(s)/
Co-Investigator |
Project Title |
|---|---|
|
Dr. Amanda Acevedo (BRP) |
Perceptual processing of pain expressions: a potential intervention target for reducing cancer pain differences |
|
Dr. Travis Hyams (DCCPS)
|
Exploring communication and resource needs of young onset colorectal cancer patients |
|
Dr. Sydney O'Connor (BRP)
|
Perceptions of and barriers to time-restricted eating for weight management |
|
Dr. Heather Platter (BRP)
|
User testing of the NCI’s Automated Self-Administered 24-hour dietary assessment tool (ASA24) respondent nutrition report–nutrient intake from dietary supplements |
Career and Training
FELLOWSHIPS: NCI Cancer Prevention Fellowship Program
Cancer Prevention fellows conduct up to four years of mentored, multidisciplinary research in cancer prevention while working in NCI programs of their choosing, including BRP. The program offers an opportunity to earn an MPH sponsored by NCI. Applicants must have a doctoral degree, or complete one before the start of the fellowship. The 2021 application cycle is open now through August 17, 2020. Visit the program website »
HBRB Program Director position
BRP welcomes letters of interest for a Program Director position in BRP’s Health Behaviors Research Branch. Qualified applicants should have a PhD or equivalent and experience in any relevant area of cancer-related health behaviors and behavior change, especially those with expertise in behavioral genomics, behavioral economics, cancer survivorship, health geography, systems science and organizational behavior, or epidemiology. Read the position description »
TCRB Program Director position
BRP invites candidates with a PhD or equivalent and experience in tobacco control research, especially in tobacco cessation, to apply to a newly approved open position to serve as a Program Director in the Tobacco Control Research Branch. Program Directors support external researchers, develop funding opportunities, conduct and present studies, and more. Read the position description »
Events
August 17—NIH webinar: Electronic Nicotine Delivery Systems Use Funding Opportunity
Tune in on August 17 at 1:00 p.m. ET for a pre-application webinar on the “Interventions to Prevent Electronic Nicotine Delivery Systems (ENDS) Use Among Adolescents” funding opportunity. Hear from multiple speakers including BRP Program Director Dr. Rachel Grana Mayne. Learn more about the webinar »
September 22—Implementation Science Consortium in Cancer Virtual meeting
The Second Annual Implementation Science Consortium in Cancer (ISCC) Meeting will be held virtually, from September 22–23, 2020. The 2020 ISCC will be a working meeting that will focus on short-term and long-term cancer control priorities; challenges and opportunities during the COVID-19; synergies and gaps in the implementation science space across NCI-funded initiatives; and discussion on infrastructures for cross collaboration. The event will feature a variety of presentations and discussion sessions, as well as pre-work to encourage more engagement and discussion during each virtual session. Register now » ![]()
September 28—NCI webinar: Perspectives on Cancer and Aging
The fourth installment of the Perspectives on Cancer and Aging: The Arti Hurria Memorial Webinar Series will be held on September 28 at 1:00 p.m. ET. Dr. Hyman Muss from the University of North Carolina School of Medicine and Dr. Grant Williams from the University of Alabama at Birmingham will be the featured speakers. Register for the webinar » ![]()
October 5—NCI webinar: Cancer and Aging Sample Grants
The fifth installment of the Perspectives on Cancer and Aging: The Arti Hurria Memorial Webinar Series will be held on October 5 at 1:00 p.m. ET. Two researchers, Dr. Michael Irwin from the UCLA Geffen School of Medicine and Dr. Supriya Mohile from the University of Rochester, shared their grant applications with the research community through the BRP Sample Grant repository. In this webinar, they will offer their perspectives on the grantee experience and insights about the grants process. Register for the webinar » ![]()
Mid-December—Dissemination & Implementation Conference
Co-sponsors NIH and AcademyHealth will host the 13th Annual Conference on the Science of Dissemination and Implementation in Health (D&I) virtually in mid-December. This year’s theme is Dissemination and Implementation Science in a Dynamic, Diverse, and Interconnected World: Meeting the Urgent Challenges of our Time. Learn more about the conference » ![]()

